What we need to learn
To safely connect a patient to a breathing machine, doctors place a breathing tube through the mouth and between the vocal cords (intubation). A breathing tube can put pressure on a patient’s voice box and cause an ulcer (similar to how shoes can cause blisters on feet). If an ulcer develops, it can cause long-term problems with breathing, speaking, and swallowing even after the tube is taken out. Some breathing tubes are smaller and some are larger. Using smaller breathing tubes may prevent these long-term problems with breathing, speaking, and swallowing, whereas using larger breathing tubes may help patients get off of the breathing machine faster.
What we are doing
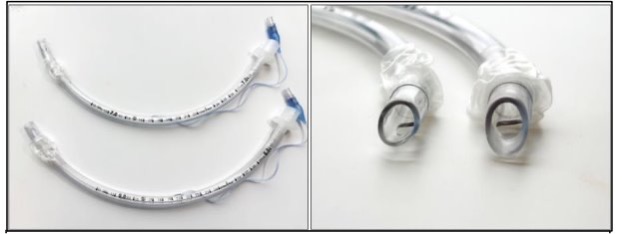
This research study is called the “Tube Size Randomized Trial during Emergency Tracheal Intubation” (BREATHE) and is funded by the Patient Centered Outcomes Research Institute (PCORI). The goal of the study is to learn whether smaller or larger breathing tubes (personalized for a patient’s height) are better for getting patients through their critical illness and preventing long-term problems with breathing, speaking, and swallowing.
Participating in this study will not impact the quality of care patients receive. For each patient:
- When the doctors feel a smaller tube would be best for a patient, they will use a smaller breathing tube, and the patient does not take part in the study.
- When the doctors feel that a larger breathing tube would be best for a patient, they will use a larger breathing tube, and the patient does not take part in the study.
- When the doctors think that both a smaller and larger breathing tube would be equally safe and effective for the patient, the patient will be enrolled in the BREATHE study and the breathing tube will be randomly chosen by the BREATHE study. If, at any time, the patient’s condition changes, doctors can change the breathing tube for whatever size they think is needed.
The study team will:
- confidentially review the patient’s medical record
- meet with the patient after the breathing tube is removed
- explain the study and ask for permission to contact the patient 6 months after breathing tube placement
- ask the patient at 6 months after breathing tube placement about his or her breathing, speaking, and swallowing
Questions
If you have any questions or concerns about this study, you may contact the Principal
Investigator, Dr. Jonathan Casey at Jonathan.d.casey@vumc.org or (615) 208-6139. If
you have questions about your rights as a research participant, or concerns or
complaints about the research, you may also contact the Vanderbilt Human Research
Protections Program at (615)- 322-2918.
Participating Sites
- Vanderbilt University Medical Center
- Denver Health
- Hennepin Healthcare
- University of Alabama at Birmingham
- University of Colorado at Denver
- University of Washington
- Wake Forest Atrium Health
Frequently Asked Questions
What is a breathing machine?
Some seriously ill patients in the hospital need help to breathe. In this situation a breathing machine – also known as a “mechanical ventilator” – is used to breathe for the patient while they heal. A breathing machine helps move air in and out of the lungs to maintain proper levels of oxygen in the blood. To use a breathing machine, the doctors and nurses insert a tube through the mouth to reach the lungs. The procedure for inserting a tube into the mouth to reach the lungs is called “intubation”.
What are the risks of each size tube?
Both smaller and larger breath tubes are used every day across the world. As far as we know today, both smaller and larger breathing tubes are equally safe and effective. However, it is possible that smaller breathing tubes could reduce the risk of injury to the voice box while larger breathing tubes help get patients through their illness and off the breathing machine faster. The goal of the BREATHE study is to determine if breathing tube size affects any of these risks.
Who is eligible for the BREATHE study?
- Adults who are receiving treatment in a participating emergency room or intensive care
- unit (ICU) whose doctors have determined that they need a breathing tube may be
- eligible for this study. Patients are eligible only if their doctors and nurses think that
- using either a smaller or larger breathing tube would be consistent with optimal care for
- them. Patients can only participate if they are undergoing breathing tube placement at a
- hospital participating in the study. They cannot volunteer at any other time.
This work is supported through a Patient-Centered Outcomes Research Institute (PCORI) Project Program Award (BPS 2024C1-37478).
Patient Partners

Patricia Wilder
has experienced both emergency and extended intubation as the result of her being shot as a young adult. After weeks of being intubated and having a tracheostomy, she had to re-learn to swallow properly and to speak normally. “Over the past 50+ years, I’ve had countless surgeries/recoveries resulting from the original injury. Advising on this project is a way for me to further patient-centered care in emergency settings and planned procedures based on lessons I have learned. I’m grateful for the opportunity.” Since April 2022, Ms. Wilder served on the Patient Engagement Panel for the ADVANCE Collaborative, a Clinical Research Network led by OCHIN. Also, she has been an active advisor on AA-CONVENE, a research study using Artificial Intelligence to identify exposure to firearm violence in EHR clinical notes. Ms. Wilder is the Patient Stakeholder for the University of Washington.
April-Lynn-Stovern
is a COVID intubation survivor who was intubated for 19 days. After 42 days of combined hospitalization and rehab, she experienced brain fog and other symptoms of long-COVID. April is passionate about supporting research that is focused on improving outcomes for patients in the critical care space and hopes to use her patient experience and healthcare journey to provide support and guidance to research teams as they work to establish best practices. “I believe this research is important because it directly impacts millions of people who rely on the ventilator for survival and my hope is this will reduce symptoms and complications with prolonged intubation. Over 3 ½ years later I still feel the effects of my intubation.” Her background in logistics and customer service also provides expertise to help guide patient-facing study components. Mrs. Stovern serves as the Patient Stakeholder for the University of Minnesota Hennepin.


Kelly Harden
brings decades of experience as both a Nurse Practitioner and educator serving as the Dean of a College of Nursing. Her personal journey as a patient and two-time transplant survivor, however, provides incredible insight into the challenges of navigating a complex healthcare system. As a committed Advisory Council member, she provides recommendations to ensure that patient facing information is accessible and that the team considers patient and caregiver perspectives. “Being a patient representative on a research study team is an honor and a responsibility. I have the privilege to bring real voices into scientific discovery and to ensure that the human side of research is never forgotten.” Ms. Harden’s personal and professional backgrounds are uniquely well suited to provide patient-centered feedback to enhance clinical care and research. Ms. Harden serves as the Patient Stakeholder for Vanderbilt University Medical Center.
Catherine Anderson
was diagnosed with airway stenosis in 2004 after two years of misdiagnosis, and her journey has included numerous surgeries and treatments, including a major airway reconstruction. She is the Founder and CEO of Living with Idiopathic Subglottic Stenosis community, now the largest global support group for airway stenosis patients. A frequent conference presenter and guest speaker, she is also an avid patient advocate with multiple publications in the clinical trial space. She brings extensive experience in patient recruitment, questionnaire design, and data interpretation centering the patient and caregiver experience. Catherine serves as a consultant to the North American Airway Collaborative at Vanderbilt University and is professionally trained in market research and works with doctors to conduct research into iSGS and its treatments. Ms. Anderson serves as an organizational representative and Patient Stakeholder, representing the larger ISS patient community.


Eileen Rubin
co-founder of the ARDS Foundation, has served as President and CEO for over two decades. An attorney by profession, Ms. Rubin experienced a life-altering diagnosis in her early 30’s that resulted in a lengthy ICU stay and long road to recovery. Today, she a well- known advocate for patients and their families serving in a variety of roles to help educate medical professionals and to improve and inform research. Ms. Rubin stresses, “Including the patient and family perspective is critical in medical research to ensure studies are designed from beginning to end with the patient in mind and with objectives focused on concerns, issues and endpoints of importance not only to advance medical research but also to include priorities of patients.“ She has served in an advisory capacity for numerous organizations including the American College of Chest Physicians, the Society of Critical Care Medicine and the American Thoracic Society. She was also the lead investigator for a PCORI Pipeline to Proposal Award. Ms. Rubin is an organizational representative and Patient Stakeholder providing insight on patient centeredness and dissemination priorities.
Sherman Transou
was an active business owner but in 2015 his life was changed when he learned that a virus was attacking his heart. Five months later he joined the growing community of transplant recipients and has embraced this opportunity to inspire and educate others in his community. With over 30 years of experience in leadership, client retention, and customer service, Sherman understands the urgency of fostering strong relationships and uses his background to prioritize the patient-centeredness of research. In addition to serving on the Board of Directors for HonorBridge, he is an active advocate, speaker and leadership coach. Mr. Transou uses his experience as a patient to help research teams effectively connect with patients and their families. Mr. Transou is the Patient Stakeholder for the Coordinating Site at Atrium Health Wake Forest Baptist.


Barbara Gould
is a COVID intubation and liver transplant survivor and has personal experience with post ICU syndrome and PTSD. As a retired social worker, Ms. Gould understands the importance of patients’ physical and mental health and has used her experience to platform the needs of patients and families. She shared, “I strongly believe that medical research saves lives and that patient representation in that process is essential.” Ms. Gould has spoken with the media about her hospitalization with COVID to raise awareness and most recently was the patient representative for the PCCRG group at the ATS 2025 International Conference. Ms. Gould serves as the Patient Investigator for the University of Colorado Anschutz and the University of Colorado Denver.
Jasmine McIntosh
is a young adult cancer survivor who is an active advocate in the community. She is passionate about health inequities and supports research that is working to improve outcomes for all patients. Her background in systems and technology is an asset to clinical trials. “I believe clinical trial research is important because for me personally, as a two-time cancer survivor with a rare gene, research has allowed me to be on the receiving end of innovative care. I am grateful for that access as not everyone has that same opportunity. Research helps to make it more accessible and to continue the work toward health equity.” Ms. McIntosh is the Patient Stakeholder for the University of Alabama, Birmingham, providing guidance on community consultations and patient facing materials.

We want to know what you think about the BREATHE study
We welcome feedback and questions from the community about research like this. If you have comments or questions please contact Dr. Jonathan Casey at Jonathan.d.casey@vumc.org or (615) 208-6139
