Doctors and scientists started the “Waveform capnography and colorimetric carbon dioxide detection during tracheal intubation of critically ill adults (WAVE)” study in April of 2025. This website provides information about the WAVE study.
The goal of the WAVE study is to determine whether one of the two methods that doctors commonly use to verify that a recently-placed breathing tube is correctly located in the windpipe (trachea) is best.
Why the WAVE Study is Needed:
When doctors care for some patients who are very sick, a plastic breathing tube is inserted through the mouth and into the windpipe (called the trachea). A breathing machine (called a ventilator) is then connected to this plastic tube to help the patient breathe.
After the breathing tube is inserted, doctors must know for sure that it is in the correct place (the windpipe). If the breathing tube was accidentally inserted somewhere else, such as the passage that carries food to the stomach (the esophagus), air won’t reach the lungs, and the patient will get worse.
Doctors watch the breathing tube during placement to be sure that it correctly enters the windpipe. To confirm that it is inserted correctly, doctors use one of two simple tests at the bedside every time a breathing tube is inserted. Both tests work in a similar way—by looking for carbon dioxide in the breathing tube. Carbon dioxide is a substance that leaves the body only through your breath when you exhale so if carbon dioxide is detected coming from the breathing tube, the doctor knows it is in the correct place.
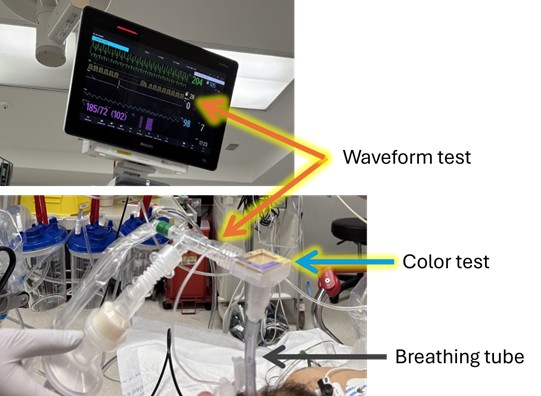
- Waveform test: One carbon dioxide test in widespread use senses the amount of carbon dioxide exhaled every breath and displays that information for the doctor as a graph (also known as a waveform) on a digital monitor.
- Color test: The other carbon dioxide test uses a special color-changing paper to show the doctor that carbon dioxide is present in each breath from the breathing tube. Both tests are approved by the U.S. Food and Drug Administration (FDA).
Both work and are used for millions of patients each year, but the study is trying to figure out if one is best.
What we are doing
Doctors and nurses are doing a research study to try and learn which carbon dioxide test is best for our patients who are very sick and need a breathing tube inserted. The study is named the WAVE Trial.
Normally a doctor would use either the waveform test or the color test right after the breathing tube is inserted to be sure it is in the correct location. In this study:
- Doctors will use both tests at the exact same time
- During and right after the breathing tube insertion, detailed information is collected on the breathing tube procedure, patient vital signs, and whether the two tests showed doctors that the breathing tube was in the correct location.
- Researchers will collect more information about the patient and the breathing tube procedure from the medical record.
The doctors and nurses will care for all patients as they normally would, including using the information from both carbon dioxide tests to deliver the best care. Participating in this study will not change the quality of care that patients receive.
Consent for Emergency Care
Placing seriously ill adults on a breathing machine is an emergency procedure. There is often no time for doctors to discuss the risks and benefits of the procedure. Patients are often unconscious or too sick to make decisions. So, doctors go ahead with life-saving care without the patient’s okay (consent).
Consent for Research during Emergency Care
For these same reasons, doctors may enroll patients in the WAVE Trial and collect information from both carbon dioxide tests after breathing tube insertion without getting the patient’s okay (consent). Important research to find the best emergency care, like the WAVE study, can sometimes be done without getting patients’ okay (consent) ahead of time through a process called “Waiver of informed consent”. Studies done with waiver of informed consent are reviewed by researchers, doctors, and an independent ethics committee.
Participating Sites
- Hennepin County Medical Center
Frequently Asked Questions
What is a breathing machine?
Some seriously ill patients in the hospital need help to breathe. In this situation a breathing machine – also known as a “mechanical ventilator” – is used to breathe for the patient while they heal. A breathing machine helps move air in and out of the lungs to maintain proper levels of oxygen in the blood. To use a breathing machine, the doctors and nurses insert a tube through the mouth to reach the lungs. The procedure for inserting a tube into the mouth to reach the lungs is called “intubation”.
Who is eligible for the WAVE study?
Adults who are receiving treatment in an emergency room or intensive care unit (ICU) whose doctors have determined that they need a breathing tube may be eligible for this study. Patients are eligible only if their doctors and nurses think that using both the color and waveform tests after intubation is safe for the patient. Patients can only participate if they are undergoing breathing tube placement at a hospital participating in the study. They cannot volunteer at any other time.
What is the difference between the waveform device and the colorimetry device?
Both are FDA approved devices that are used every day by doctors to make sure the breathing tube is correctly positioned in the windpipe.
- The waveform device checks the air that passes through the breathing tube so that it can measure how much carbon dioxide (CO2) is present. This amount of CO2 is then shown on a medical monitor as a graph of CO2 in the air going in and out of the breathing tube over time. The doctor can look at the graph and see the CO2 levels rise as air leaves the lungs and fall as fresh air enters the lungs.
- The colorimetry device connects to the breathing tube and has a small piece of litmus paper that changes color when the level of CO2 changes. When air leaves the lungs the paper turns yellow, and when fresh air enters the lungs the paper turns purple.
What are the risks of each test?
There are no known risks to either method of confirming the location of the tube. Both are used every day across the world. Normally your doctor would use either the waveform method or the colorimetry method to check tube location. In this study, your doctor used the information from both methods to confirm the tube is in the correct location.
Why are you sharing information about this trial with the community?
The goal of the WAVE trial is to produce information that helps patients, families, and doctors choose the best test to check breathing tube placement so that patients can receive the best care possible. Making sure that patients, families, and community members know about the study and its findings is important to achieving this goal.
We want to know what you think about the WAVE Trial
We welcome feedback and questions from the community about research like this. If you have comments or questions please contact Dr. Brian Driver at brian.driver@hcmed.org or call him at 612-873-7448.